Extraction And Drying Of An Aqueous Solution Lab Report

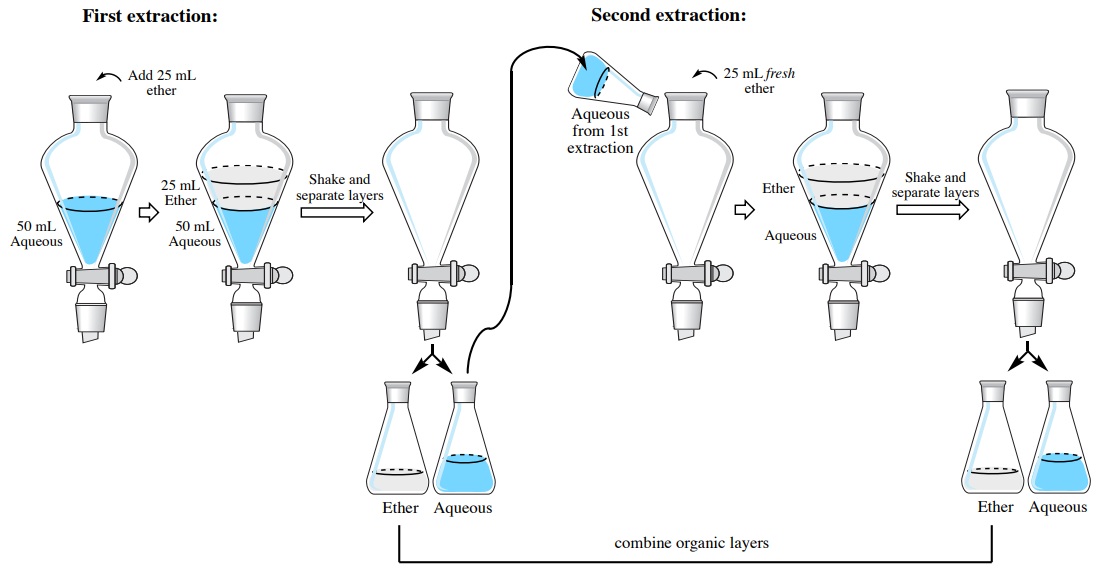
Wash the ether layer with 10 ml of a saturated aqueous sodium chloride solution.
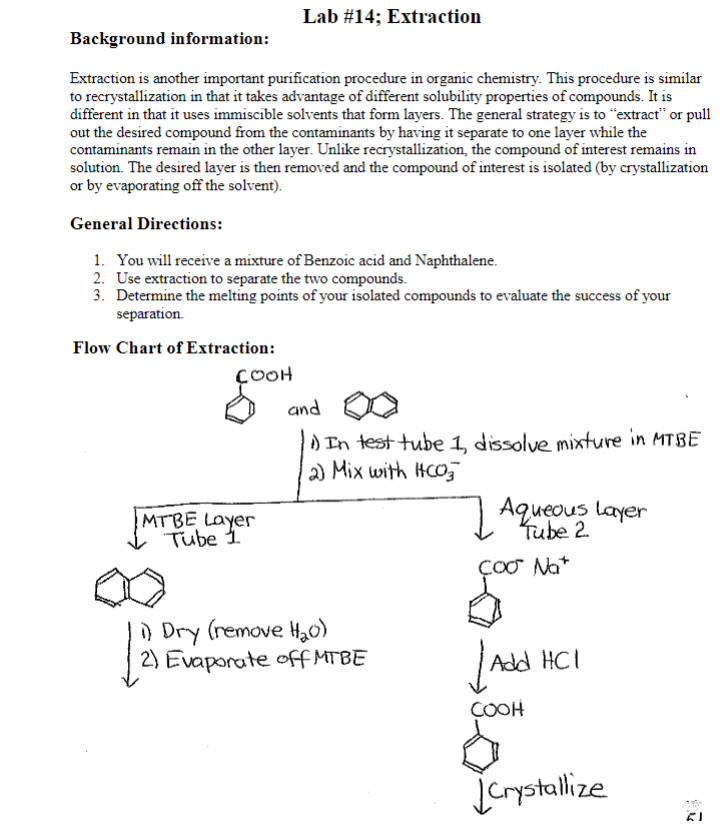
Extraction and drying of an aqueous solution lab report. With each extraction the top dye solution became closer to the original unmixed color for sudan blue while the bottom layer remained orange in the receiving beaker. For this reason naoh was selected to be the aqueous solution to be used in the extraction procedure. Aqueous solution was cooled in the ice bath until a solid of acetylsalicylic acid formed. When performing an extraction two immiscible solvents must be used.
The solid washed with 5 ml of cold water after being collected with vacuum filtration. A second extraction was performed with 20 ml of cold 10 sodium hydroxide solution in the separatory funnel and the bottom aqueous layer was drained. For the second part of the week one experiment clear separation was found between the two dyes. See experiment 4 for a solvent miscibility chart.
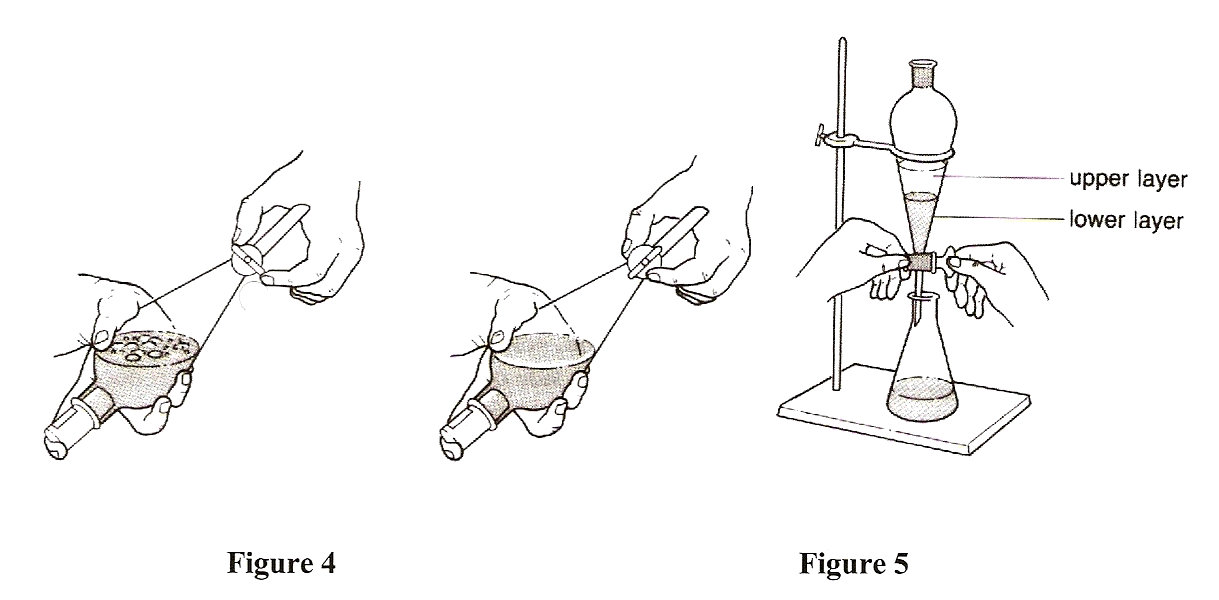
When two immiscible solvents are mixed the less dense solvent makes up the top layer while the more dense solvent makes up the bottom layer. Instructor may demonstrate this principle as part of the pre lab discussion. Combine this aqueous wash layer with the previous aqueous extraction layers.