Discussion Extraction And Drying Of An Aqueous Solution
When two immiscible solvents are mixed the less dense solvent makes up the top layer while the more dense solvent makes up the bottom layer.
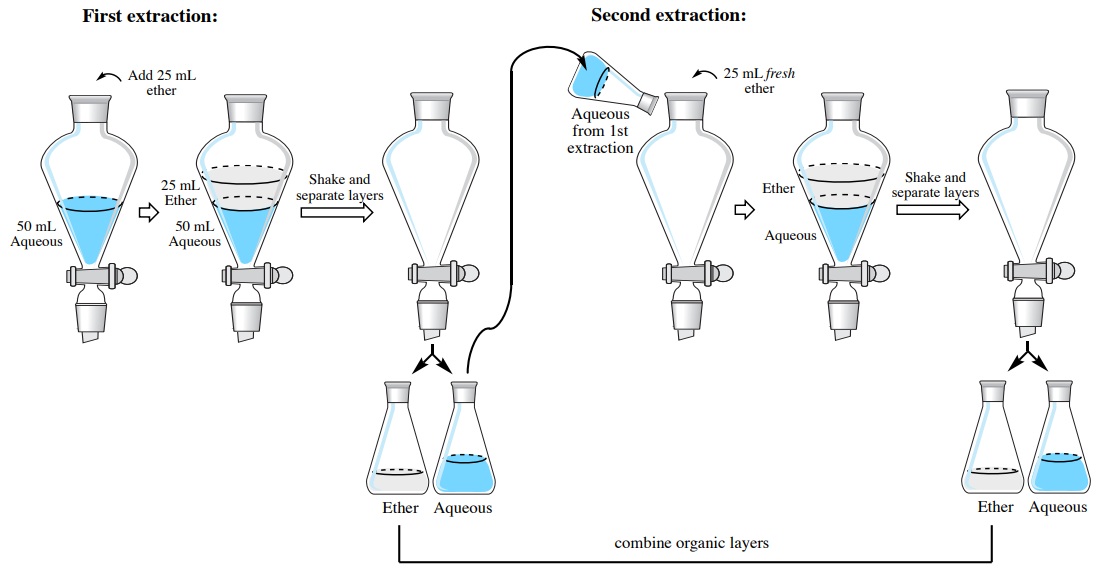
Discussion extraction and drying of an aqueous solution. If the drying agent remains undissolved after 15 minutes then discard the aqueous solution still in the 250 ml beaker down the drain. Allow the layers to separate discard the aqueous layer make sure which is which and transfer the ether solution to a clean dry 50 ml erlenmeyer flask. 2 the handbook covers both vacuum and gravity filtration techniques. The inversions were done slowly in order to see the extraction stepwise.
See experiment 4 for a solvent miscibility chart. Dry the organic layer that is left at this point by adding approx. Explain why vacuum filtration is the method of choice for separating the benzoic acid from the neutralized aqueous solution. Progress of the extraction of methyl red the colored compound from the acidic aqueous layer bottom into the organic layer top.
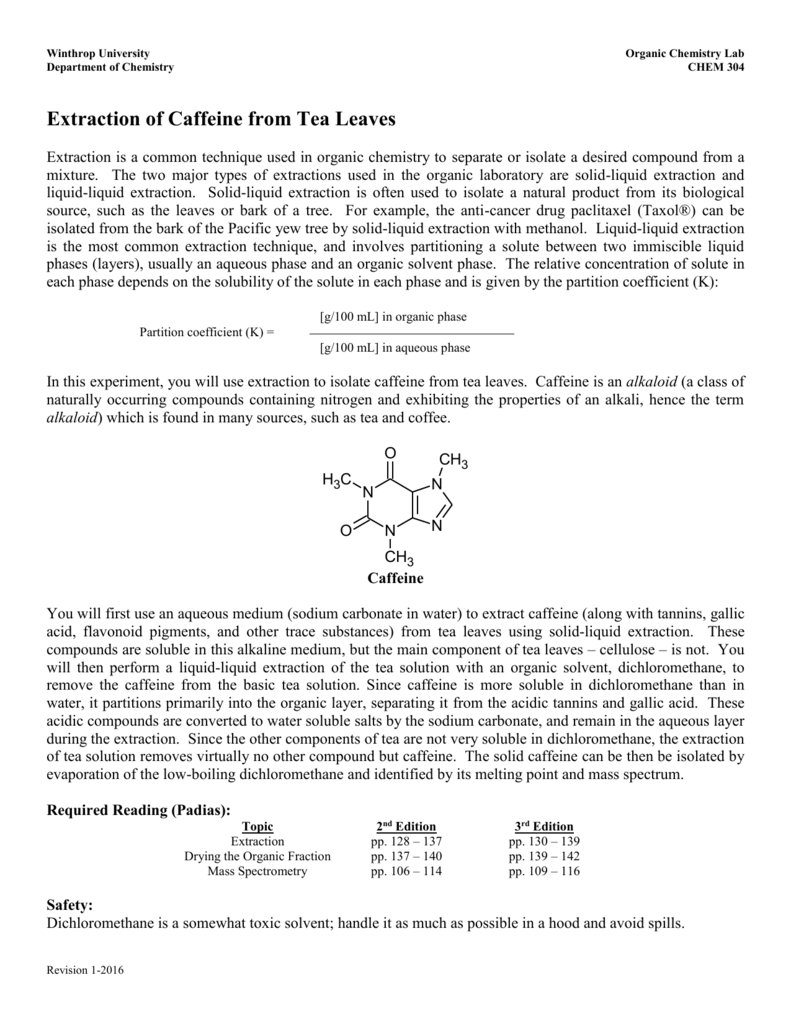
Discussion and conclusion in this experiment a mixture with a 1 1 1 ratio of basic acidic and neutral components will be dissolved in diethyl ether and then isolated and extracted into its corresponding components by an extraction procedure. Organic compounds are often extracted from aqueous solutions using a separatory funnel and a hydrocarbon solvent such as hexane. Flask as the distillation flask. If the drying agent forms a clump at the bottom of the tube then more drying agent is needed.
Hydroxide solution and combine these two aqueous extracts in another labeled flask caution. With even gentle mixing the methyl red extracts rapidly. 4 5 g of anhydrous magnesium sulfate to the liquid contained in an erlenmeyer flask for a discussion of drying. An extraction procedure works on the ability to manipulate solubilities of solvents in different conditions which in this experiment is the solvents.
Add drying agent until the organic solvent is sufficiently dried of aqueous solvent. Solid liquid extractions are often used to extract natural compounds from natural sources such as plants 1. When performing an extraction two immiscible solvents must be used. Set up a simple distillation apparatus with a 100 ml r b.
Add a small amount enough to cover the bottom of the flask of anhydrous sodium sulfate na 2 so 4 to the ether solution to absorb any residual water. Single extraction figure 4 20. Old drying agent and add fresh drying agent to the filtered solution if it becomes wet looking or clumped. If you are uncertain check with the instructor.
Elizabeth valcourt chem 546 02 26 september 2017 extraction results and discussion. A drying agent such as magnesium sulfate can be used to further extract aqueous solvent from the organic solvent after extraction.